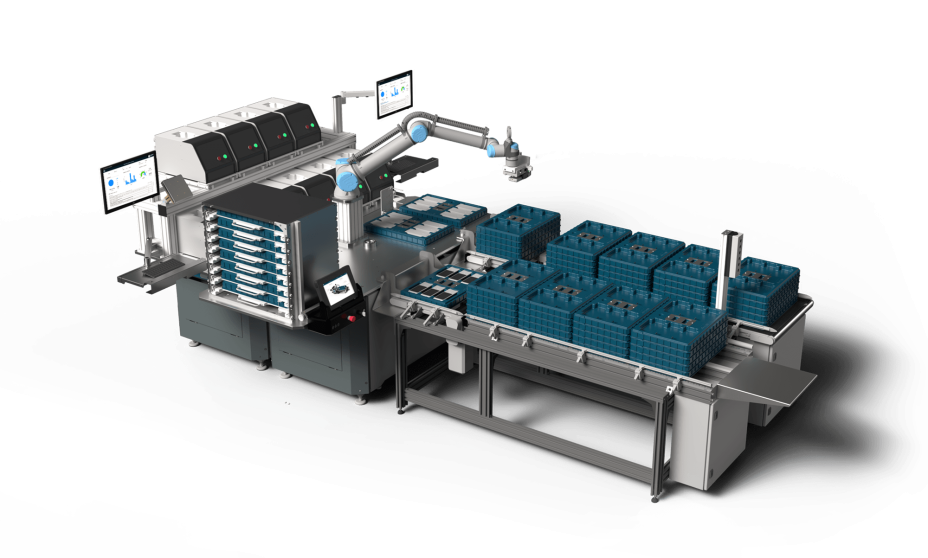
Medical devices need to fulfill highest standards in terms of functionality and safety. With ESSERT Robotics, you can automate the programming and testing of medical devices – for a streamlined and reproducible workflow.
Medical device
testing
Program and test medical devices for maximum reliability