The MicroFactory
platform
The Future of robot automation.
Unleash the power of modular Robotics for next-level manufacturing for Pharma and MedTech industries.
The Future of robot automation.
Unleash the power of modular Robotics for next-level manufacturing for Pharma and MedTech industries.

On each module a specific process can be replicated to meet our customers’ specific requirements.
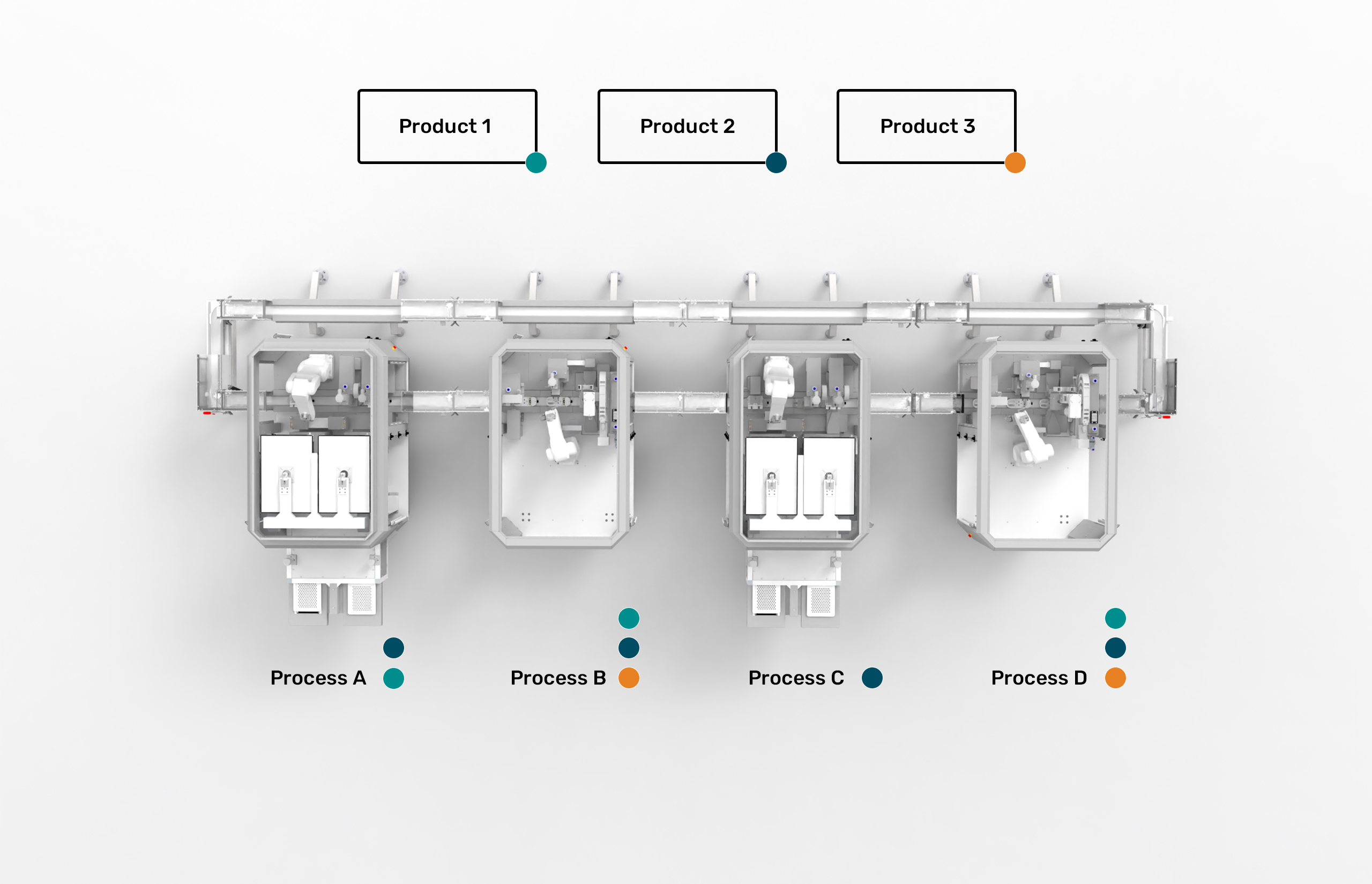
The MicroFactory automation platform is process oriented, not product oriented.
Different products have similar manufacturing processes in common. Use one MicroFactory process module for various products and save money.
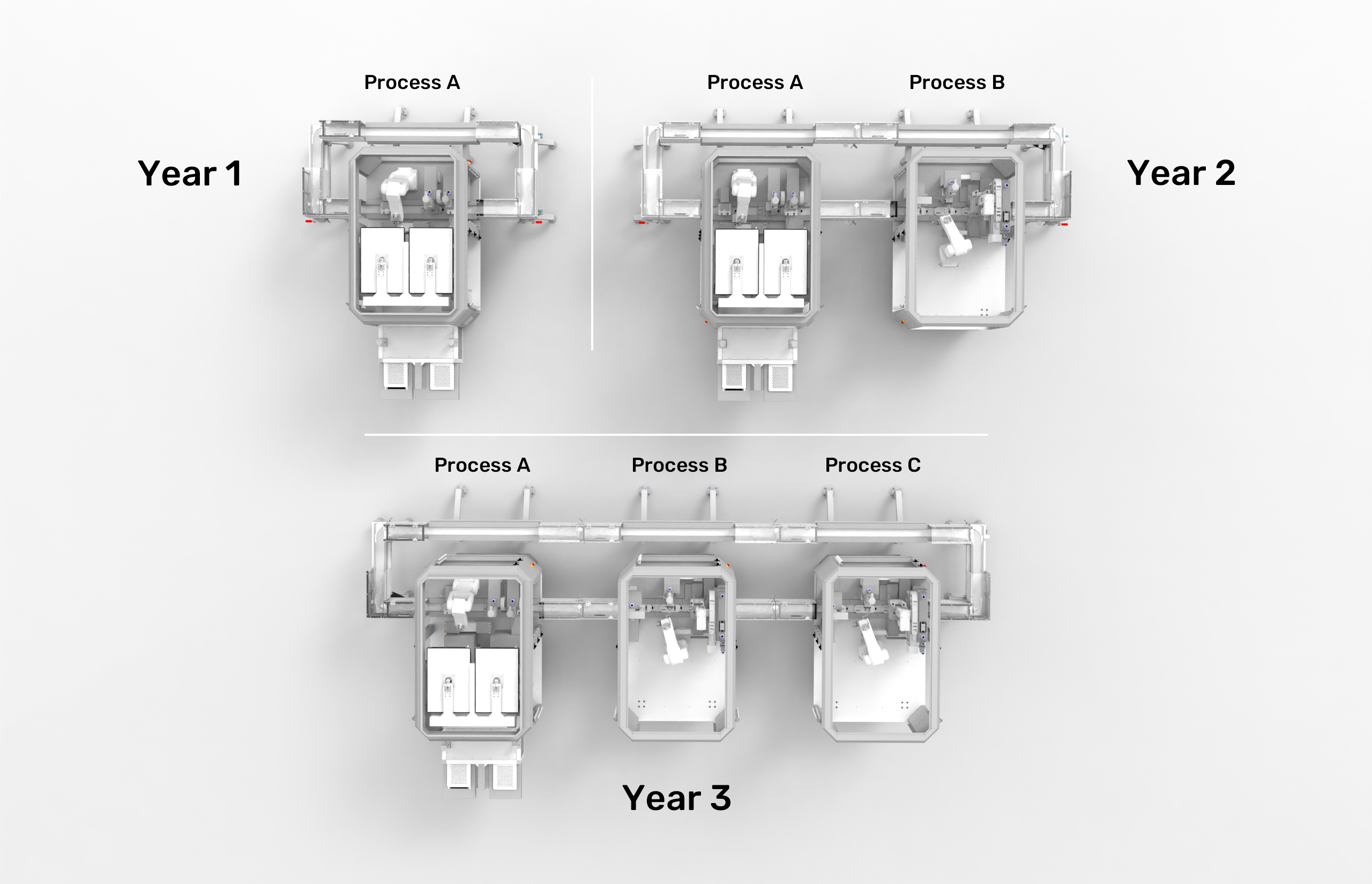
Start with one process module for a specific good manufacturing process. Gradually add automation to your production as needed.
The ability to expand your production line over time, including modular documentation and qualification of individual process modules, opens up a new world of endless possibilities.
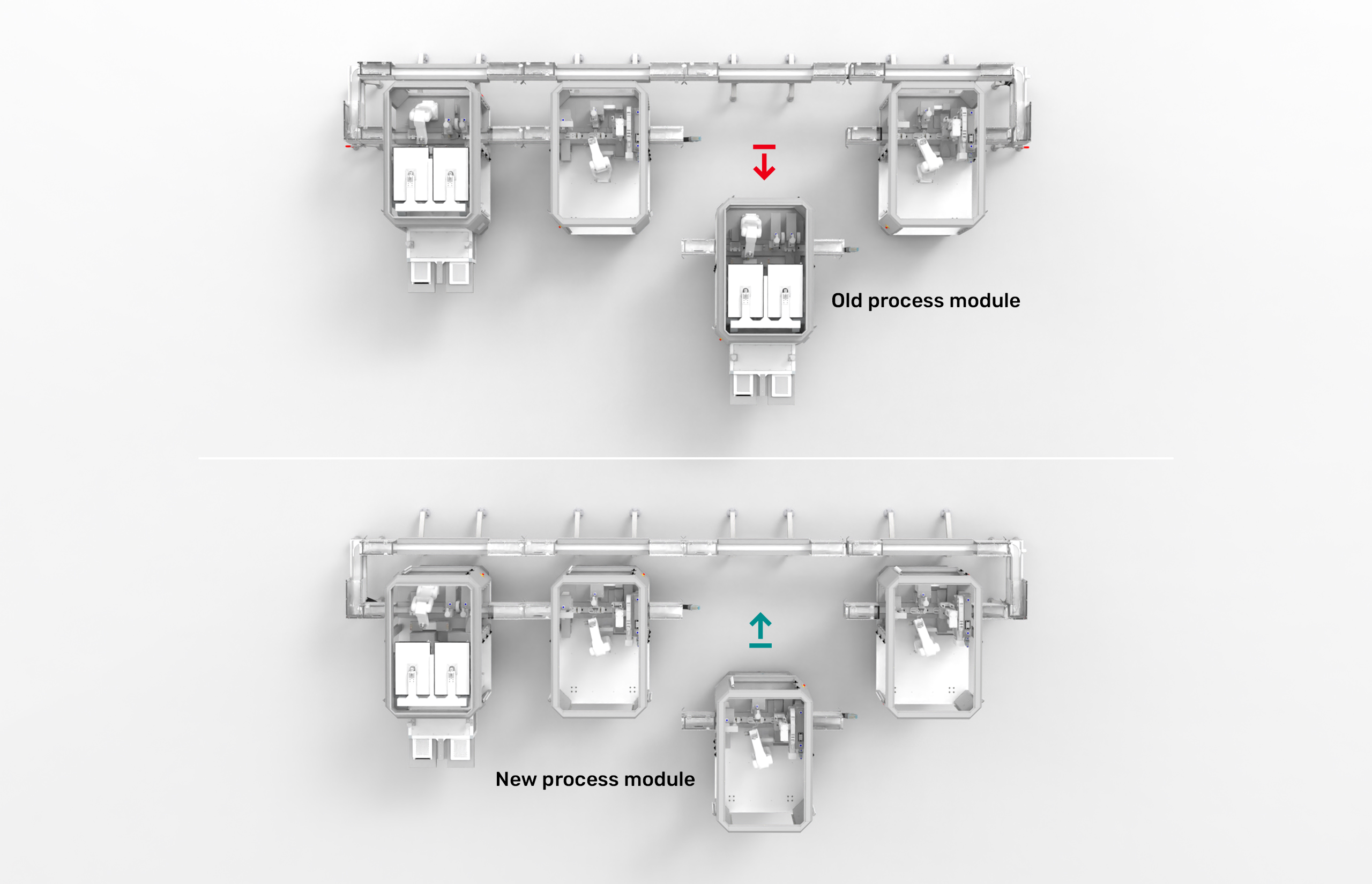
The modular architecture of the MicroFactory platform allows for seamless reconfiguration at any time.
Simply replace individual process modules with new ones to meet changing production needs. Process modules already in place remain fully validated.
Just plug & produce!
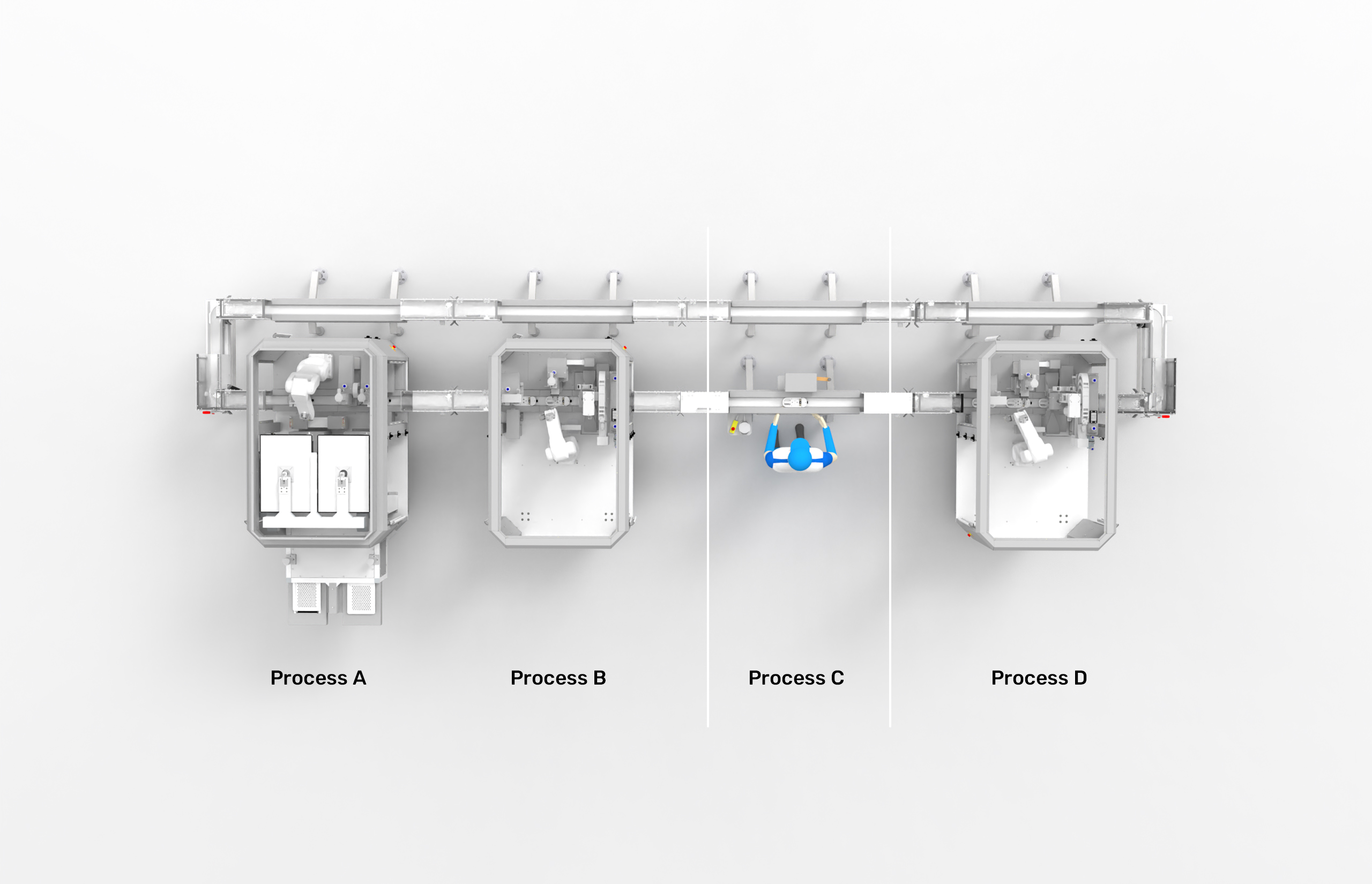
Keep your employees involved in certain production processes.
It's never been easier to integrate manual workstations into a Life Science automation line! If required, our individual MicroFactory modules are also 100% cooperative.
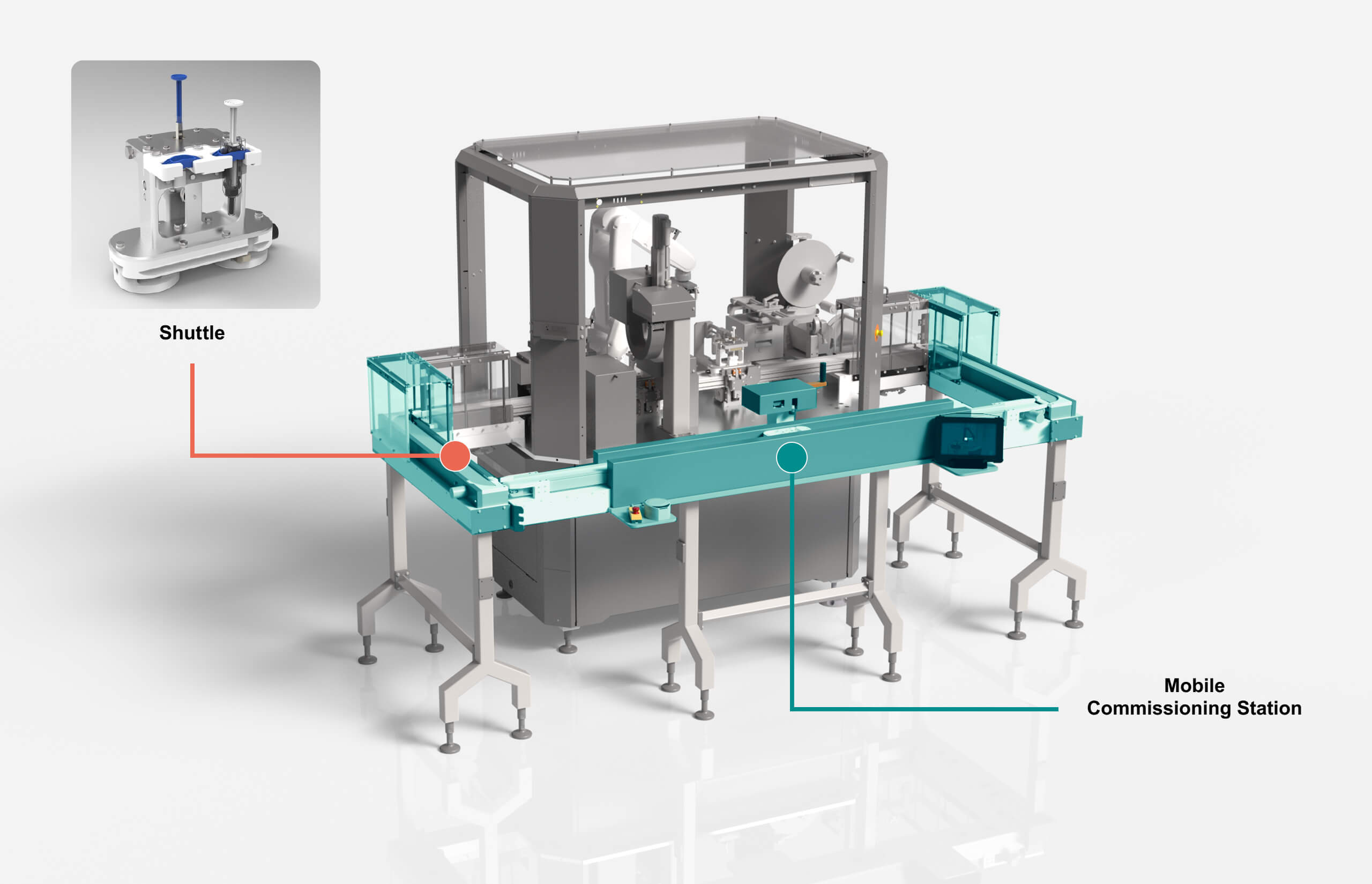
Stand-alone commissioning for short delivery times.
All MicroFactory modules are tested, fully commissioned and individually qualified using our mobile commissioning station.
No on-site assembly, very short commissioning times and a modular qualification and documentation approach facilitated by a high level of standardization.
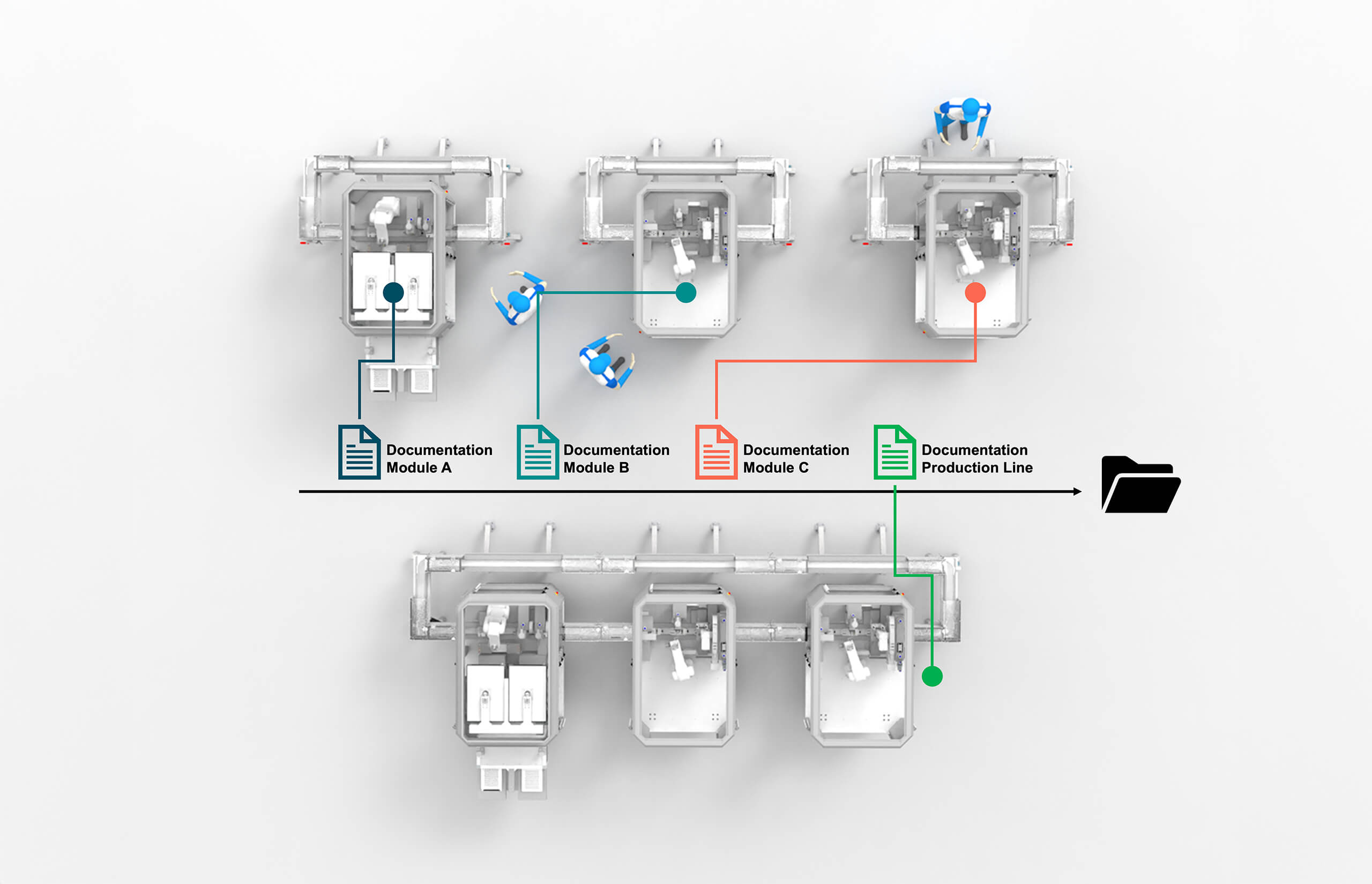
We are taking modularity to a new level and applying it to documentation, too!
The modular approach to qualification and documentation, facilitated by a high level of standardization, allows a fully qualified acceptance test to be carried out for each process module separately.
Only after individual acceptance is complete the MicroFactory line is composed for performance runs and final acceptance.

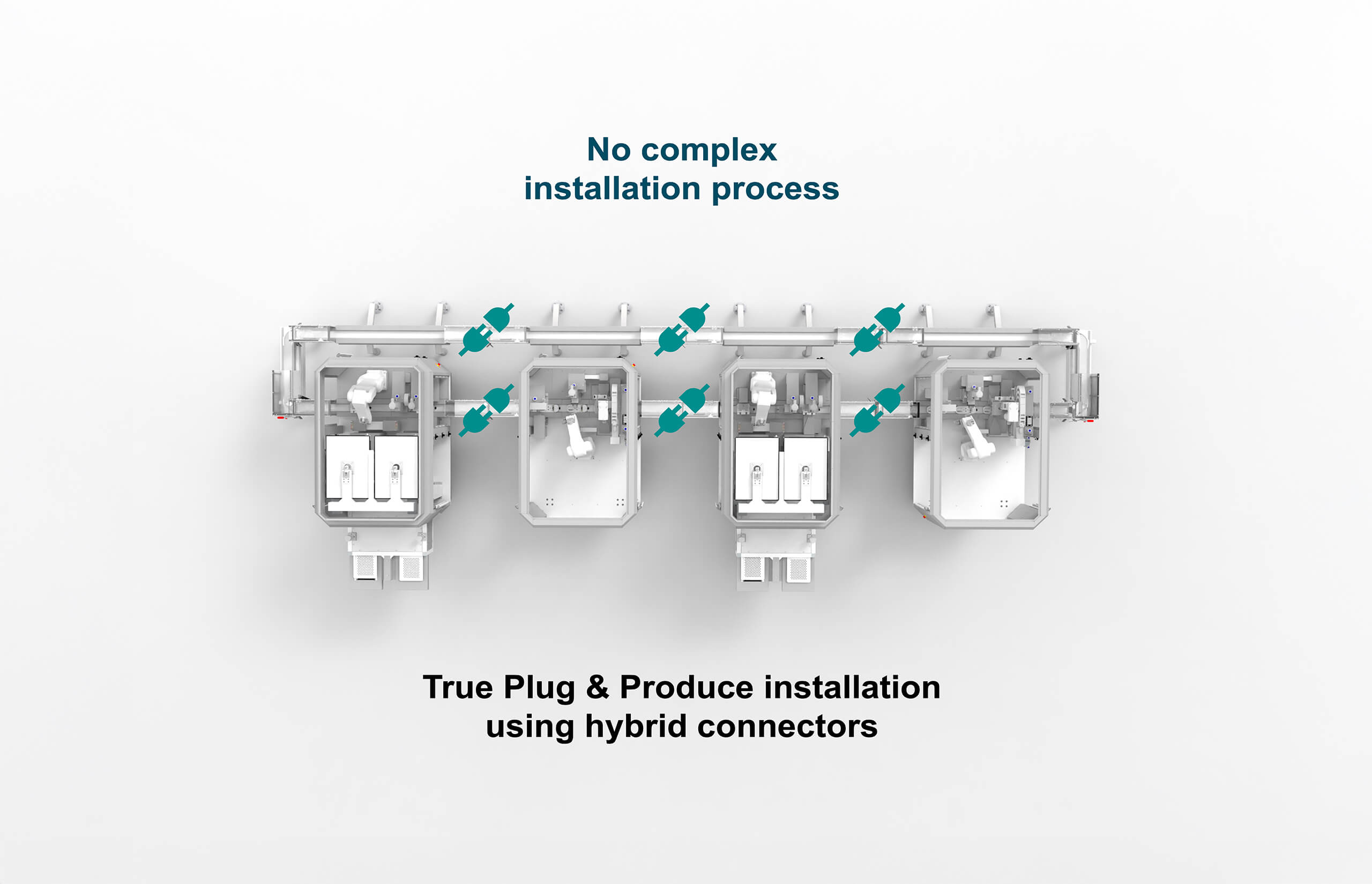
All the media required for the MicroFactory line is supplied from module to module using hybrid connectors. Only one main media supply is required for the line, making installation fast and efficient.
The entire installation qualification (IQ) can be moved to the FAT as there is no need for electrical or mechanical installation on site.
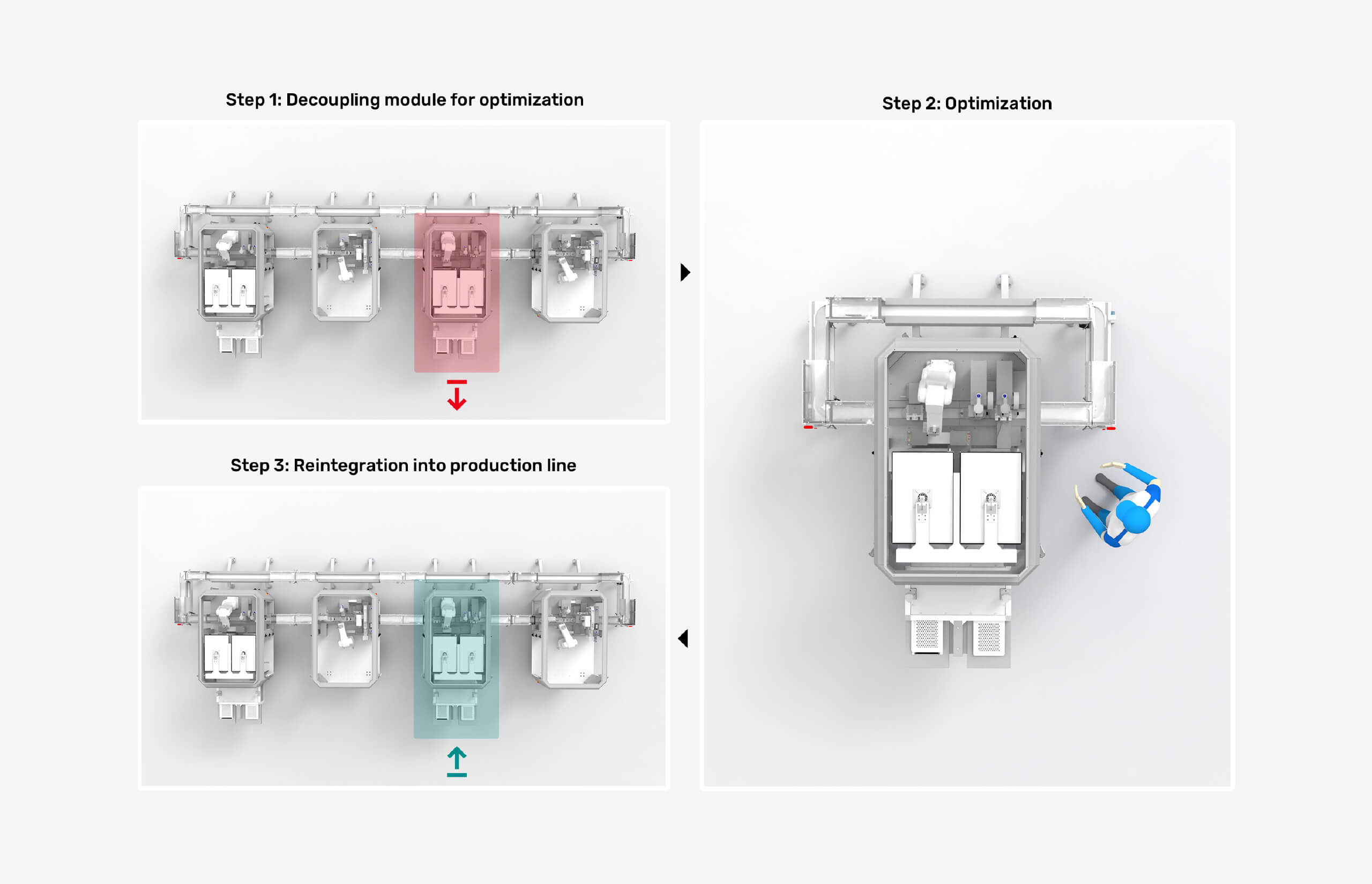
Boost operational efficiency by focusing on optimizing individual processes.
MicroFactory modules offer a unique advantage: they can be easily removed from the line for standalone process optimization. This makes it simple to implement new products or optimize inline quality monitoring.
Best of all, untouched process modules remain qualified!

The ESSERT Process Control Center (PCC) is the beating heart of our automation platform, a vital hub that seamlessly consolidates all the crucial data of all ESSERT automation systems.
With the integrated Performance Dashboard, you can monitor all process modules in real time and visualize relevant Data in one central location.
You always have full visibility into your production data.
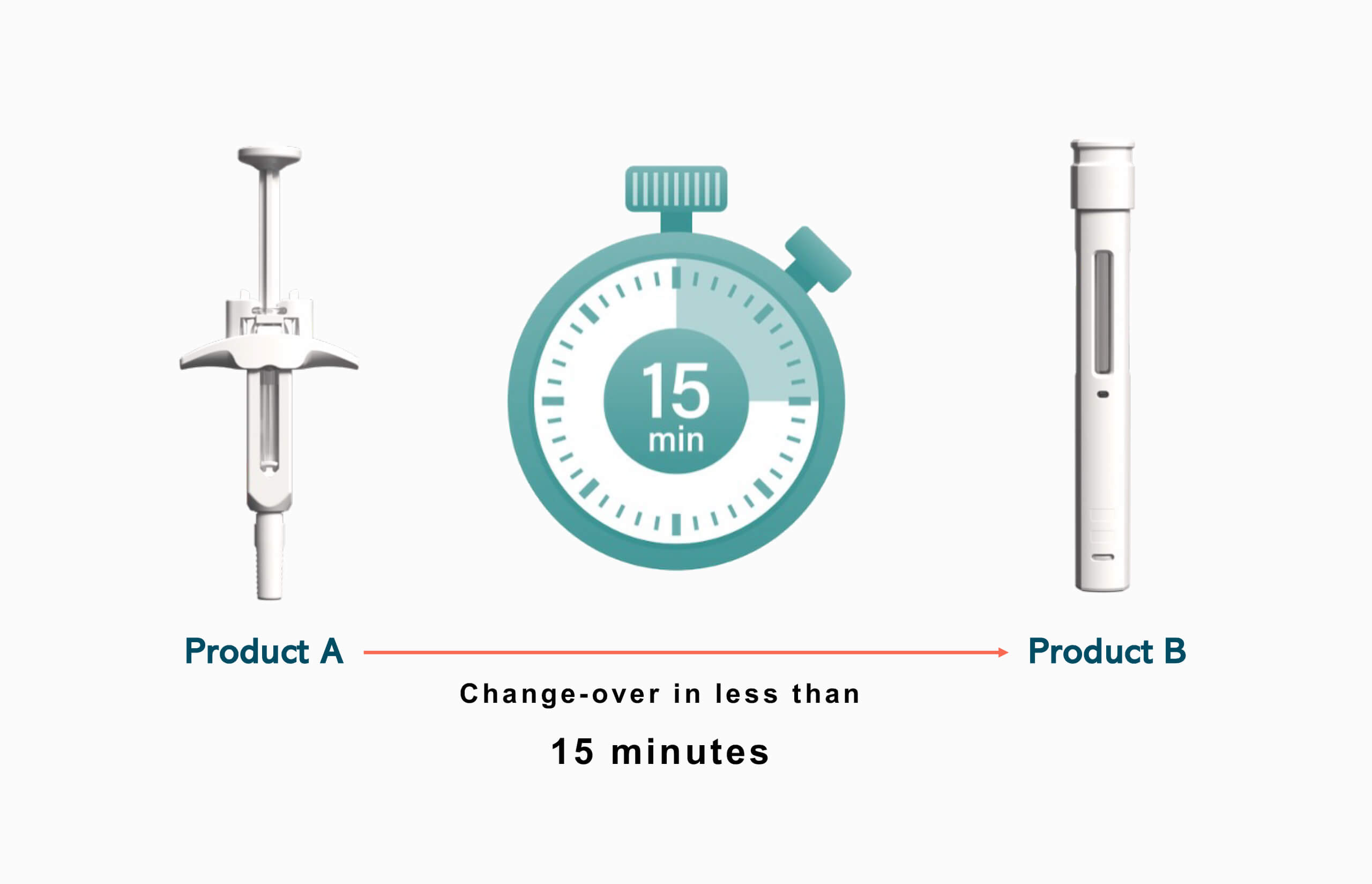
Thanks to our software-based approach and consistent use of advanced robotics, MicroFactory modules are able to change to a new product configuration fully automatically.
The operator can focus solely on clearing the line and material handling, and the next batch is ready for production in minutes.
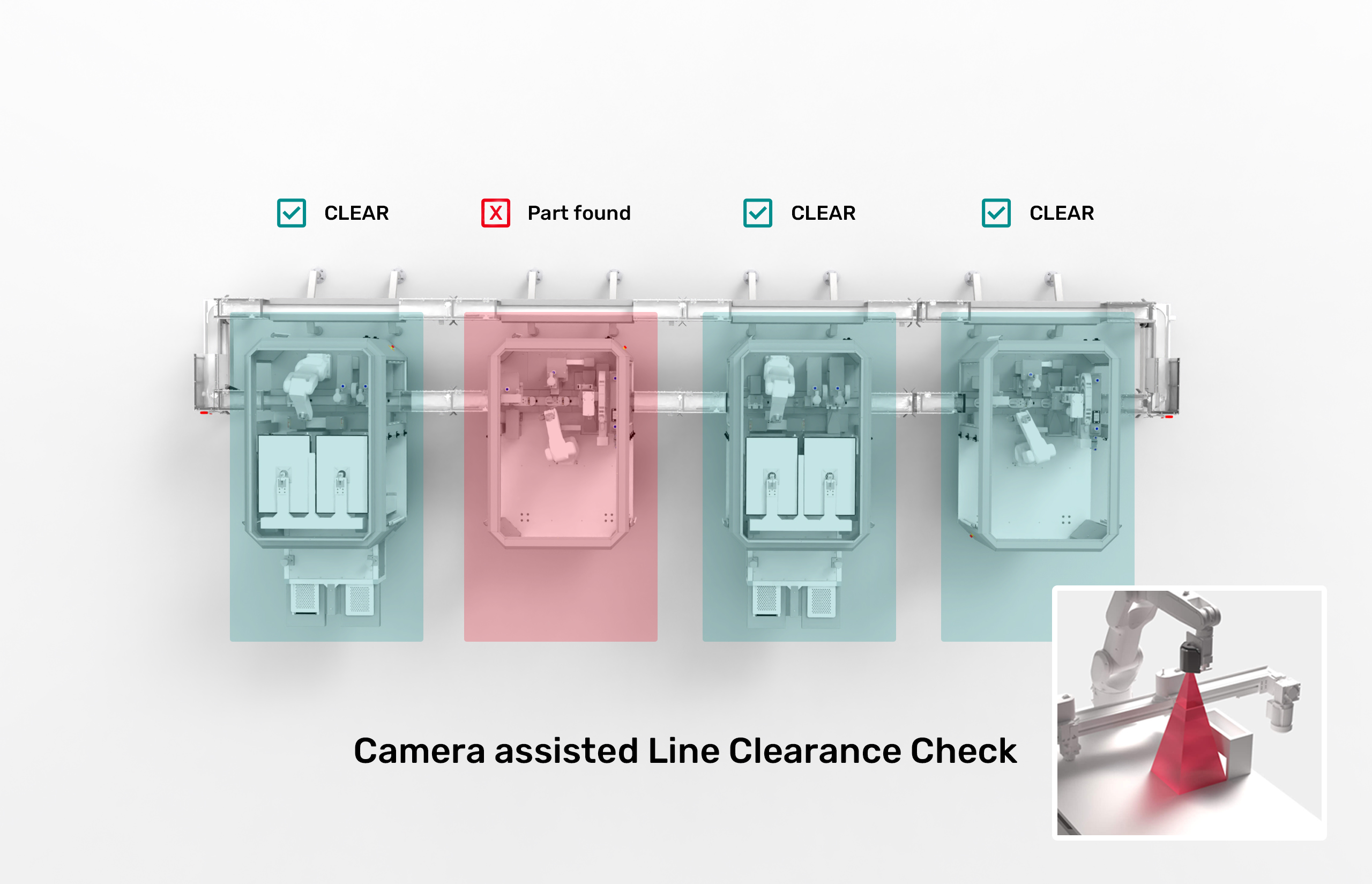
Need to make sure the line is clear of parts before and after producing a batch? Well, more eye pairs are better than one.
Our modules use intelligent camera technology to automatically perform a line clearance check after each batch production and notify you of a positive result.
Discover our scalable MicroFactory solution for syringe and PEN assembly in variable batch sizes as well as high format variance without setup operations.
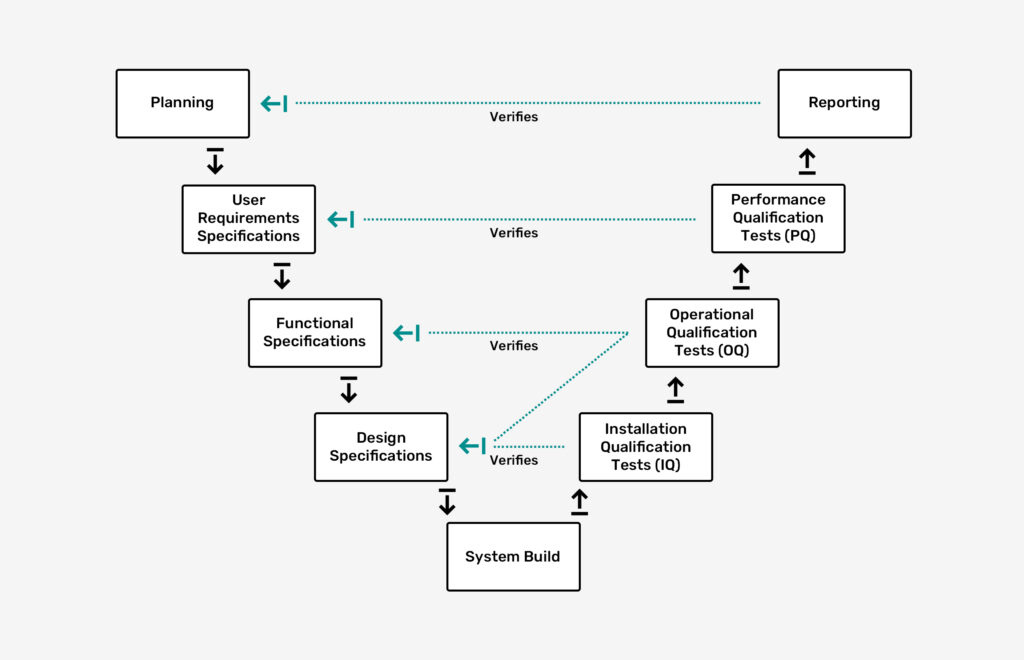
We adhere to GMP and GAMP guidelines and provide comprehensive documentation, validation, and qualification.
Our services include traceability matrix, risk analysis, Failure Modes and Effects Analysis (FMEA), design documentation, qualification, CE conformity assessment, audit trails, change control, training documentation, internal audits, Standard Operating Procedures (SOPs), deviation management, and data integrity to ensure full compliance and customer satisfaction.
Blog