Examples of medical devices
Medical devices play a crucial role in modern healthcare, encompassing a wide array of tools and equipment designed to diagnose, treat, and manage various medical conditions. From diagnostic imaging machines like X-rays and MRIs to life-saving devices such as pacemakers and defibrillators, these innovations are instrumental in improving patient outcomes and enhancing quality of life.
In this article, we will discuss different definitions of medical devices and the classes they are categorized in, before listing some medical devices that are frequently to be encountered. Also, we will take a brief look at medical device manufacturing and ESSERT Robotics’ contributions to this sector.
When is a product a medical device?
The term “medical device” is not to be used arbitrarily; instead, there are strict regulations when a product may be referred to as a medical device. Accuracy in deciding when the term applies is essential to determine the regulatory controls that are to be kept in mind along their supply chain, as special medical device regulations have been established to ensure the safety and effectiveness of these products – including premarket approval processes and postmarket surveillance.
The classification of a product as a medical device depends on several factors, including its intended purpose. Regulatory agencies such as the World Health Organization (WHO), the Food and Drug Administration (FDA), and the European Medicines Agency (EMA) play key roles in defining medical devices, but also in medical device classification and medical device regulation.
What WHO, FDA and EMA have to say on the subject
Here’s what the World Health Organization, the EMA (European Medicines Agency), and the U.S. FDA (Food and Drug Administration) say defines a medical product:
Medical devices include all the health technologies (except for vaccines and medicines) required for prevention, diagnosis, treatment, monitoring, rehabilitation and palliation. They are indispensable for universal health coverage, monitoring wellbeing and addressing outbreaks or emergencies.
Source: WHO1
Medical devices are products or equipment intended for a medical purpose. In the European Union (EU) they must undergo a conformity assessment to demonstrate they meet legal requirements to ensure they are safe and perform as intended. They are regulated at EU Member State level, but the European Medicines Agency (EMA) is involved in the regulatory process.
Source: EMA2
A medical device is an instrument, apparatus, implement, machine, contrivance, implant, in vitro reagent, or other similar or related article, including any component, part, or accessory, which
is –
- recognized in the official National Formulary, or the United States Pharmacopeia, or any supplement to them,
- intended for use in the diagnosis of disease or other conditions, or in the cure, mitigation, treatment, or prevention of disease, in man or other animals, or
- intended to affect the structure or any function of the body of man or other animals, and which does not achieve its primary intended purposes through chemical action within or on the body of man or other animals and which is not dependent upon being metabolized for the achievement of its primary intended purposes.
Source: FDA / Food, Drug, and Cosmetic Act 21 U.S.C. 321(h)3
Types of medical devices
Medical devices encompass a wide range of equipment, instruments, and tools utilized in healthcare settings to diagnose, treat, or prevent medical conditions. These devices are classified into different categories based on their intended use, level of risk, and potential impact on patient safety.
Regulatory agencies like the WHO, EMA, and FDA provide guidance documents for the classification of medical devices. Regardless of some minor differences, devices are typically categorized into classes based on their potential risks and intended use.
Class I devices: These devices are low-risk – examples include tongue depressors, thermometers, and stethoscopes.
Class II devices: Moderate-risk devices – examples include infusion pumps, blood pressure monitors, pre-filled syringes, and autoinjectors.
Class III devices: High-risk devices – examples include pacemakers, implantable defibrillators, and prosthetic heart valves.
Examples – list of frequently used medical devices
Medical equipment serves crucial roles in diagnosis, treatment, and patient care across various medical specialties. Here, we highlight a selection of commonly encountered medical devices, before discussing the particular roles of pre-filled syringes and autoinjectors in more detail.
- Autoinjectors – devices designed to deliver a single dose of a particular medication, typically for self-administration by patients, often used for medications such as epinephrine for severe allergic reactions
- Blood glucose monitor – a device used to measure and monitor glucose levels in the blood, commonly used by individuals with diabetes to manage their condition
- Blood pressure monitors – crucial tools for measuring and monitoring blood pressure levels, essential for cardiovascular health assessment
- Breast implants – implants used for breast augmentation or reconstruction following mastectomy or breast deformities
- Brackets for dental braces – orthodontic devices used in dentistry to align and straighten teeth, typically made of metal, ceramic, or plastic and affixed to the teeth to apply pressure over time to correct alignment issues
- Catheters – flexible tubes inserted into the body for various medical purposes, including draining fluids, administering medications, or visualizing internal structures during medical procedures
- Contact lenses – corrective lenses worn directly on the eye’s surface to improve vision, replacing traditional eyeglasses for vision correction
- Drug administration devices – devices such as inhalers, nebulizers, syringes, autoinjectors, and insulin pumps designed for delivering medications in precise doses and formulations
- Heart valves – implantable devices used to replace damaged or diseased heart valves, restoring normal blood flow and cardiac function
- Hospital beds – vital equipment for patient care and comfort in healthcare facilities, providing not only orthopedic support during recovery and treatment
- Pre-filled syringes – syringes pre-filled with a specific medication dose, ready for immediate use, offering convenience and accuracy in medication administration
- Pregnancy tests – diagnostic tools used to detect the presence of human chorionic gonadotropin (hCG) hormone in urine or blood, indicating pregnancy
- Prosthetics – artificial limbs and human body parts designed to restore mobility and function for individuals with limb loss or limb impairment
- Stethoscopes – iconic devices employed by healthcare professionals to auscultate heart, lung, and bowel sounds for diagnostic purposes
- Stimulators – implantable or external devices used to deliver electrical or magnetic stimulation to nerves or muscles for therapeutic purposes
- Test kits – assorted kits containing diagnostic tests for various medical conditions, providing rapid and accurate results for healthcare providers, such as blood glucose monitors and in vitro diagnostic medical devices
- Ventilators – life-saving devices providing mechanical ventilation to patients with respiratory failure or compromised breathing function
- Wheelchairs – mobility aids providing support and independence for individuals with mobility impairments, facilitating movement and participation in daily activities
Pre-filled syringes
Pre-filled syringes are particularly useful in personalized medicine, where precise dosing and customization are paramount. These syringes come pre-loaded with a specific dosage of medication, offering ease of administration and reducing the risk of dosing errors and contamination.
They are a well-established alternative to vials, which often contain multiple doses of a drug product. Especially in the case of not frequently used medications (e.g. in personalized medicine), pre-filled syringes may lead to less spoilage and thus product loss compared to vials, which ultimately can also lower product costs for patients.4
However, their manufacturing poses challenges. Ensuring sterility during production is critical to prevent contamination and maintain drug efficacy. Furthermore, medical device manufacturers have to deal with overall complex assembly processes revolving around pre-filled syringes, e.g. when equipping them with a needle-shield safety device. This, however, has become a fairly common feature of modern syringes, as the risk of needle stick injuries for healthcare workers is an issue that needs to be addressed.5
Autoinjectors and PENs
Autoinjectors and PENs are indispensable devices in modern healthcare, offering a convenient and user-friendly method for self-administration of medications. They play a crucial role in managing medical conditions such as diabetes, rheumatoid arthritis, and multiple sclerosis. Autoinjectors deliver pre-measured doses of medication subcutaneously or intramuscularly, providing patients with greater independence and control over their treatment regimens.
The interest in developing autoinjectors dates back more than 30 years ago. An early example was the use in migraine treatment, where self-administration was considered a flexible and thus preferable approach for quick and patient-friendly drug administration. Especially the rise of biologics in the 2000s, though, has led to an increase in autoinjector request, as it is especially useful in chronic disease therapy. Today, important producers include Becton, Dickinson and Company, and Ypsomed Holding AG.6 7
Nevertheless, their manufacturing presents challenges, particularly regarding precision engineering, assembly, and ensuring sterility. Medical device manufacturers must adhere to stringent quality standards and regulatory requirements to ensure the safety, reliability, and efficacy of autoinjectors and PENs in healthcare delivery.
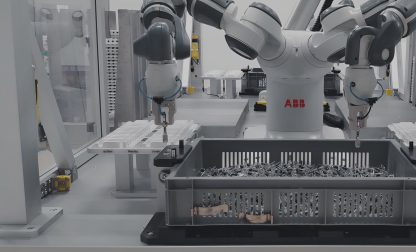
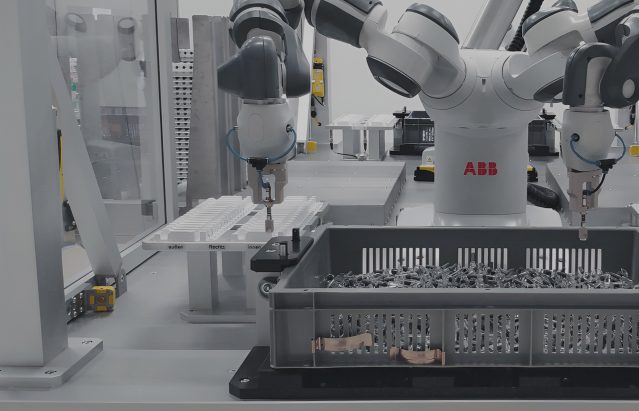
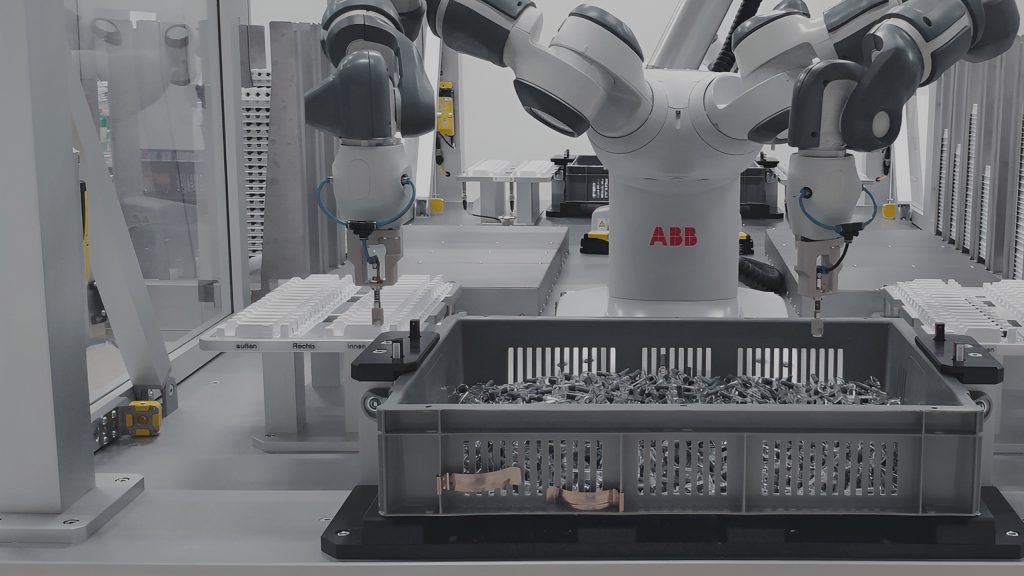
Manufacturing medical devices – facing challenges with ESSERT Robotics
Manufacturing medical devices requires adherence to stringent standards to ensure patient safety and product efficacy. But that’s not all: There is an increasing trend towards batch sizes becoming smaller yet more diverse, which poses new challenges to pharmaceutical companies and CDMOs.
With customized medical devices like prefilled syringes and autoinjectors becoming more and more important in different treatment plans, conventional machines are often no longer efficient enough. After all, changeovers in rigid systems, if possible at all, may come with considerable delays and require laborious interventions.
To support pharma companies and CDMOs in facing the challenges resulting from HMLV manufacturing of medical devices, ESSERT Robotics has developed the ESSERT MicroFactory – an innovative modular automation solution.
According to our “one process – one module” approach, the ESSERT MicroFactory comes as an extremely flexible production line, with individual modules each carrying out one specific step in manufacturing a medical device, but also being able to perform the entire production process on its own.
New modules (e.g. for labelling or bin-picking) can be directly implemented into the MicroFactory, and (dis-)activated according to the product type desired at the moment. Thanks to our standardized platform, the modules combine robotic solutions by market leaders like Fanuc, ABB, or Stäubli.
Due to the modular system architecture of the ESSERT MicroFactory, manufacturers are able to easily adapt to a large variety of different products with no format parts, minimal complexity, and changeover times of less than 15 minutes. In addition to being easily scalable by adding further process modules, this flexibility makes the ESSERT MicroFactory a future-proof and customer-friendly medical device assembly line.
- WHO: Health products policy and standards. https://www.who.int/teams/health-product-policy-and-standards/assistive-and-medical-technology/medical-devices/nomenclature. ↩︎
- EMA: Medical Devices. 04.2024. https://www.ema.europa.eu/en/human-regulatory-overview/medical-devices. ↩︎
- FDA: Information Sheet Guidance For IRBs, Clinical Investigators, and Sponsors. 2006. https://www.fda.gov/files/about%20fda/published/Frequently-Asked-Questions-About-Medical-Devices—Information-Sheet.pdf. ↩︎
- Makwana S, et al.: A. Prefilled syringes: An innovation in parenteral packaging. Int J Pharm Investig. 2011. doi: 10.4103/2230-973X.93004. PMID: 23071944; PMCID: PMC3465144. ↩︎
- Sengel B. et al: Occupation-Related Injuries Among Healthcare Workers: Incidence, Risk Groups, and the Effect of Training. 2021. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8101271/. ↩︎
- McGowan M: 30 Years in the Making – an Inside Perspective on the Emergence of Autoinjectors. ONdrugDelivery Magazine. 2019. https://www.ondrugdelivery.com/30-years-in-the-making-an-inside-perspective-on-the-emergence-of-autoinjectors/. ↩︎
- Markets and Markets: Autoinjectors Market by Usage (Reusable, Disposable), Technology (Manual, Automatic), Therapy (Rheumatoid Arthritis, Diabetes, Anaphylaxis), Route of administration (SC, IM), Volume (<3ml, >3ml), End User (Home Care, Hospitals) – Global Forecast to 2028. https://www.marketsandmarkets.com/Market-Reports/autoinjector-market-173991724.html ↩︎