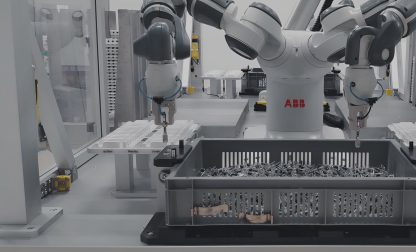
Steps in manufacturing a medical device
When manufacturing medical devices, steps vary immensely, depending on the very product to be obtained. However, medical devices share some characteristics that set them apart from conventional consumer goods; high standards in terms of regulations, quality, and safety are to be fulfilled. This is already challenging in mass production environments, and even more intricate in high mix / low volume (HMLV) manufacturing.
In this article, we are going to investigate what exemplary steps in medical device manufacturing might look like, not overseeing the phases that have to be passed before a medical device can actually enter the market.
5 Phases in medical device development
There is a huge variety of different medical devices, each designed for specific purposes. This also illustrates how individual their manufacturing steps are – from building an X-ray machine to producing a pacemaker. Nevertheless, their journey from conception to market involves common processes to ensure safety, efficacy, and compliance.
The stages set by the FDA (U.S. Food and Drug Administration), for instance, outline a systematic approach to medical device development:
- Device discovery and concept: The inception of a medical device begins with identifying a need or opportunity in healthcare. This stage involves brainstorming ideas, conducting market research, and conceptualizing the device’s functionality and purpose.
- Preclinical research: Prototype: Once the concept is defined, the development moves to the preclinical phase. Here, prototypes are built and tested in laboratory settings. The focus is on evaluating the device’s safety, efficacy, and performance before advancing to human trials.
- Way to approval: After successful preclinical testing, the medical device undergoes a regulatory approval process. This depends on the class to which a medical device belongs; the FDA distinguishes three types, based on the potential risk a device may pose to a consumer.
- Review by the FDA: Upon submission, the FDA conducts a thorough review of the application to assess the device’s safety and effectiveness. This review may involve clinical trials data, laboratory testing results, and risk assessments. The FDA ensures that the device meets regulatory standards and is safe for patient use.
- Safety monitoring after market entry: Even after a medical device receives regulatory approval and enters the market, safety monitoring remains a critical aspect. Manufacturers are required to monitor the device’s performance, collect post-market data, and report any adverse events or safety concerns to regulatory authorities. Additionally, announced or unannounced inspections at the manufacturing site are possible and, making data integrity and transparency even more crucial.1
Manufacturing steps – prefilled syringe as exemplary medical device
Prefilled syringes represent a prominent example of a medical device: They are a significant pillar in drug delivery, offering a convenient and precise way to administer medications.
Once all the components of a prefilled syringe are available at the manufacturing site – from the syringe barrel containing the drug substance to plunger rods, backstoppers, and flanges – they are subjected to a set of standardized manufacturing steps. In the following, we are going to take a closer look at these steps as performed by an ESSERT MicroFactory, highlighting how syringe assembly can be improved via automation in medical device manufacturing.
Feeding of components
Following the setup of the workpiece carrier, the next critical phase involves introducing syringe components into the production line. This step demands precision to ensure the proper alignment of all components required for assembly.
Automated systems play a vital role in seamlessly integrating syringes and attachments into the manufacturing process. Quality checks are conducted to verify accurate placement, ensuring a smooth transition to the subsequent stages of syringe production.
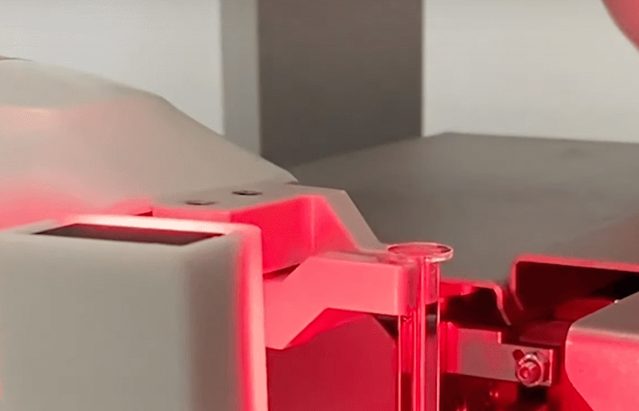
Laser labeling
Laser labeling is another crucial step in the production of prefilled syringes. Laser technology is employed to imprint essential information such as batch numbers, expiration dates, and product codes onto the syringe surface. This non-contact labeling method offers high precision and durability, ensuring clear and permanent labeling without compromising the integrity of the syringe material.
Laser labeling enhances traceability and regulatory compliance, providing essential information throughout the syringe’s lifecycle. Quality checks are conducted to verify the accuracy and legibility of the labels before proceeding to the next manufacturing phase.
Syringe assembly
Now, the manufacturing process advances to the key stage of syringe assembly. Here, the prefilled syringe components loaded on the carrier come together.
Automated medical device assembly ensuring precise alignment and secure connection of the barrel, plunger, and needle. This assembly step demands a high level of accuracy and cleanliness to guarantee the integrity of the final product. Again, quality control measures are rigorously applied, confirming that each syringe is flawlessly assembled before proceeding to subsequent manufacturing stages.
Discharge of finished syringes
Upon completion of the manufacturing process, the final step involves the discharge of finished syringes from the production line. This includes the efficient and orderly removal of syringes that have undergone assembly, marking, and quality checks. Automated systems carefully handle the discharge process, maintaining the integrity and cleanliness of the syringes.
Medical device testing and packaging
After the completion of the manufacturing phases, the prefilled syringes undergo rigorous testing and packaging procedures. Comprehensive assessments are conducted to verify the functionality, safety, and quality of each syringe. Various testing methods, including performance checks and sterility assessments, are performed to ensure compliance with regulatory standards.
Once the testing phase is successfully completed, the prefilled syringes are meticulously packaged. Automated systems like the ESSERT MicroFactory facilitate the precise and secure packaging of each unit, safeguarding it against external elements and ensuring its integrity until it reaches the end-user. Quality control measures are applied throughout the testing and packaging processes to guarantee the delivery of safe and reliable medical devices to healthcare settings.
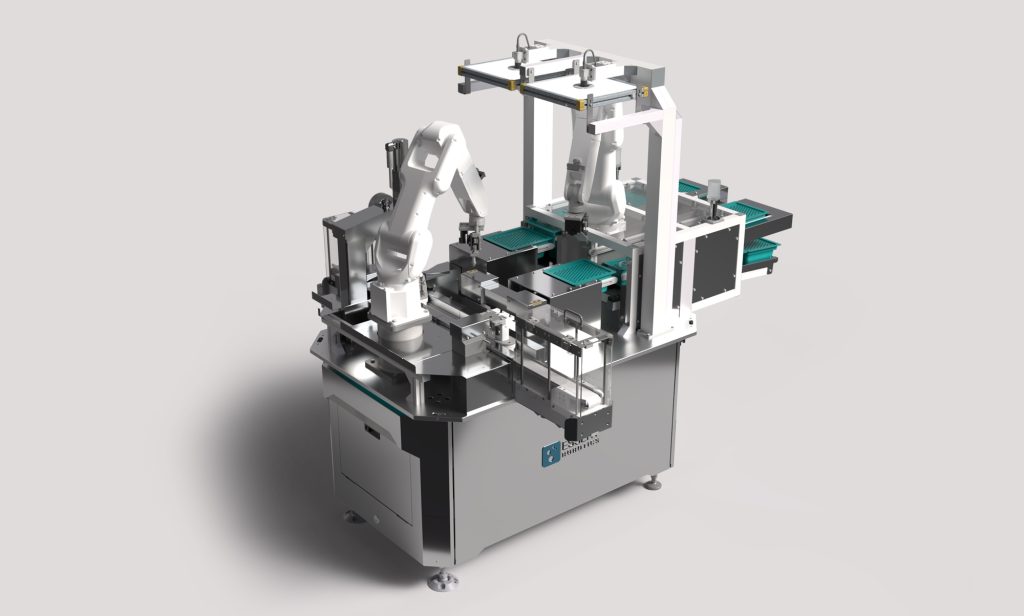
Automated medical device manufacturing in the ESSERT MicroFactory
To sum up: Automated medical device manufacturing in the ESSERT MicroFactory offers unparalleled advantages in the production of medical devices. With its smart, modular, and scalable production line, the MicroFactory represents an efficient and flexible manufacturing solution tailored to the specific needs of high mix/low volume requirements.
By minimizing setup time and offering autonomous commissioning of process modules in a medical device assembly line, the MicroFactory ensures rapid changeovers between different products. Additionally, its ability to integrate manual workstations allows for seamless integration of manual processes when needed.
On the other hand, the ADVANCED Robotic Workstations that a MicroFactory is composed of may come pre-validated and can easily be integrated into the flexible production line – which also facilitates the transition from development to commercial manufacturing.
With precise and error-free production, reliable 24/7 operation, and minimal changeover time, the ESSERT MicroFactory provides a cost-effective solution for medical device assembly, helping manufacturers increase production quality and save valuable time.
- https://www.fda.gov/patients/learn-about-drug-and-device-approvals/device-development-process ↩︎