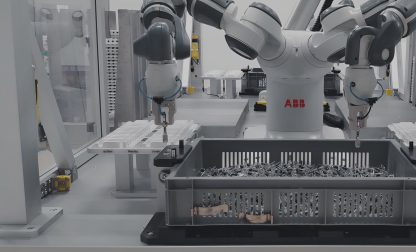
Medical device assembly line: ESSERT MicroFactory of the future

Medical device assembly is a critical aspect of the life sciences industry, requiring precision, flexibility and innovation to meet evolving market demands. As the industry moves towards personalized products and unique market segments, the challenges of High Mix / Low Volume (HMLV) production become more pronounced. Changes in the product pipeline are leading to reduced batch and order sizes and an increasing importance of customized devices.
This article highlights the hurdles to be faced in medical device manufacturing and shows how the innovative modular system architecture of the ESSERT MicroFactory allows easy adaptation to a wide range of medical products, such as pre-filled syringes or auto-injectors. It requires minimal complexity and no format parts, while ensuring high OEE through fast changeover times for efficient High Mix / Low Volume manufacturing.
Medical device assembly – the challenges to master in HMLV production
The challenges to be mastered in HMLV production are numerous and require both strategic and technological innovation. Changes in the product pipeline are resulting in reduced batch and order sizes with greater variability.
This also has an impact on production costs, which have to be kept reasonable in order for manufacturers to stay competitive. However, the frequent changeovers in HMLV manufacturing may become a major issue for conventional systems and show the limits of standard equipment. Their several format parts make changeovers a complex and time-consuming task, ultimately resulting in fewer items produced by the end of the day.
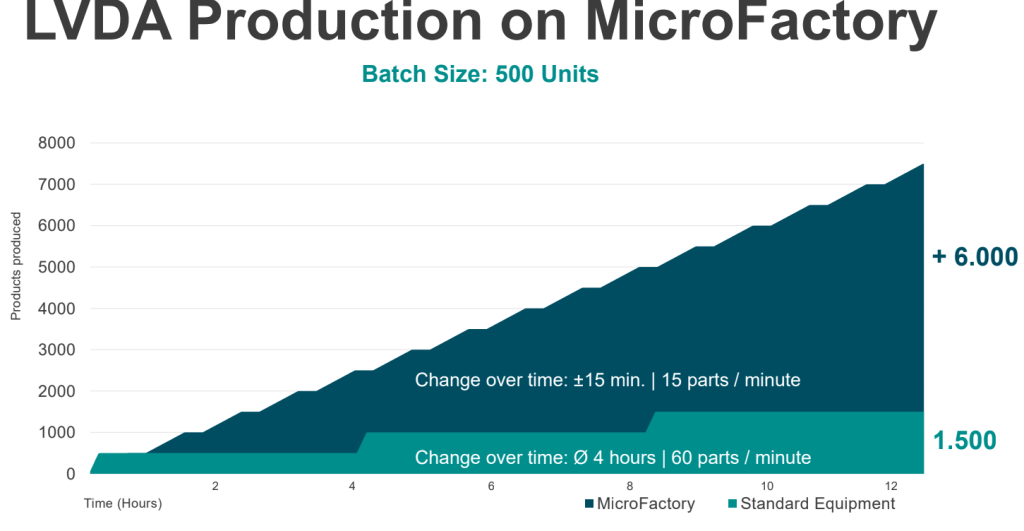
Rethinking existing processes is as necessary as establishing technologies that can keep the pace of volatile HMLV production environments. Flexible automated solutions like the ESSERT MicroFactory emerge as promising solutions, with the chart below highlighting the possible gains in efficiency at an exemplary batch size of 500 units.
Automation to increase safety, quality, and efficiency
Automation has proven to be a viable approach in mastering the aforementioned challenges related to HMLV manufacturing – e.g. responding to the trend towards smaller volumes and diverse configurations. ESSERT Robotics has therefore developed a flexible, modular, and scalable production line: the MicroFactory.
The innovative modular system architecture allows easy adaptation to a wide range of medical products, such as prefilled syringes or autoinjectors. It comes with minimal complexity and no format parts, while ensuring high OEE through fast changeover times of less than 15 minutes. This is further stressed by the chart above, showcasing the increased efficiency that can be achieved in the production of multiple small batch sizes.
Automation in medical device manufacturing powered by the ESSERT MicroFactory ensures consistent, reliable manufacturing procedures, meeting stringent quality standards. And with the ability to quickly adapt to changing production requirements and even switch between different product types, automation can make HMLV manufacturing more efficient and cot-effective.
One process – one module
The ESSERT MicroFactory’s “one process – one module” approach offers a high level of flexibility compared to traditional machinery. Unlike rigid systems, the MicroFactory allows for effortless customization, as it consists of multiple individual modules.
By analyzing complex production environments and breaking them down to their individual steps, workflows can be simplified and become less complex. Consequently, each process within a manufacturing line is assigned to one specific module when designing a customized ESSERT MicroFactory.
The reduced complexity also facilitates the adaptation or addition of process modules, enhancing overall flexibility and scalability. But also right from the beginning, the MicroFactory can be designed for catering to diverse product types, enabling it to effortlessly switch between different production lines. The respective process modules are activated according to the chosen production line – a syringe in the case of the graphics below.
Meanwhile, additional modules remain inactive until a changeover in the production line is required – e.g. when switching from syringe to PEN production. The changeover can be initiated either via software input or automatically, according to the components that are fed into the production line. Once this process is started, the single modules are (dis-)activated, exchanged and reconfigured. That way, the new production line can be hosted by the same platform, with changeover times of less than 15 minutes.
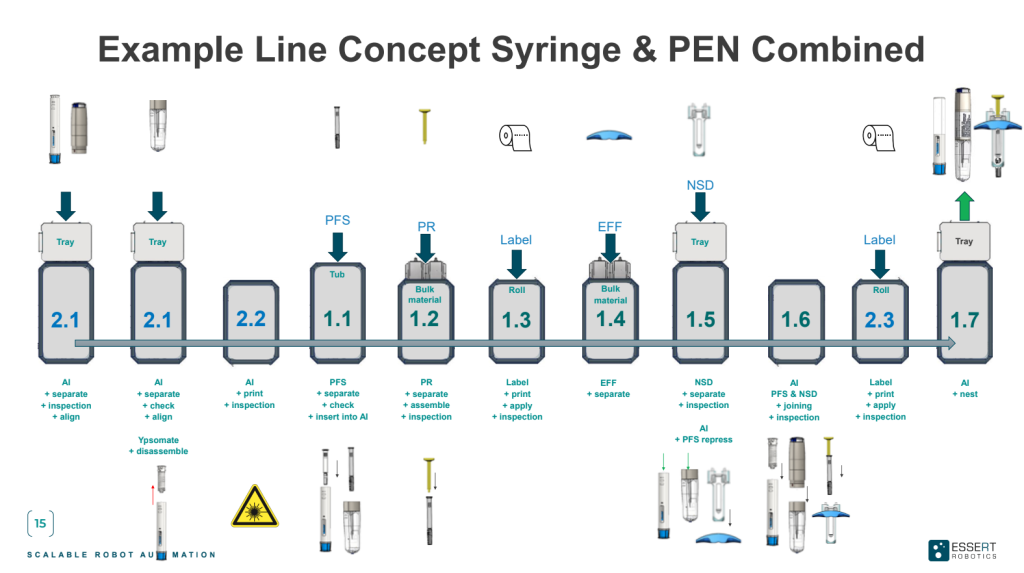
Various products in one medical device assembly line
Unlike traditional, rigid manufacturing systems, the modular ESSERT MicroFactory allows seamless adaptation for diverse product ranges. Considering the increasing need for customized medical devices, which are produced in smaller batches but greater variability, such flexible systems are crucial for quickly adapting to changing product types and requirements.
This flexibility enables manufacturers to efficiently produce various medical devices, from syringes to auto-injectors, on a single assembly line. Each standardized process module is crafted for a specific task, ensuring rapid product transitions with minimal setup time, complexity, and no format parts. Changeovers can be performed via software commands, with advanced sensor technology supporting to adjust to changing manufacturing environments, minimizing downtimes and ensuring high OEE.
It is even possible to design MicroFactories that perform changeovers in complete autonomy, including the substitution, reconfiguration and reassembly of individual modules. This was the case for Roche, powering an ESSERT MicroFactory to assemble both syringes and autoinjectors on one assembly line.
The peak of robotic innovation on one platform
ESSERT Robotics introduces an innovative platform that seamlessly converges solutions by industry leaders like Fanuc, ABB, and Stäubli. But instead of individually implementing and configuring these solutions at the manufacturing site, ESSERT Robotics has chosen a Plug & Produce approach: Each process module is fully configured and set up before delivery. At the manufacturing site, these modules simply have to be positioned and assembled via plug connection, allowing customers to get started with medical device assembly as easily and quickly as possible.
The Process Control Center acts as a central hub, facilitating collaboration among diverse robotic systems. Through standardized hardware and software, ESSERT fosters direct communication among modules, which can even work independently.
The modular setup of an ESSERT MicroFactory also facilitates maintenance: The user-friendly connections enable manufacturers to easily dis- and reconnect individual modules. These can even be temporarily replaced by a human workforce, as the MicroFactory allows the integration of a manual workstation, which might also come in handy when a specific process is too complex or costly to be automated.
Selective pre-series validation – total compliance
The capability of individual modules to execute entire workflows allows for pre-validation of processes, independently or collectively. That way, manufacturers can selectively pre-validate individual process steps and eventual adaptations within the production line without the entire manufacturing process being reassessed.
Due to the MicroFactory’s modular architecture, the pre-validated new or modified process modules can effortlessly be integrated. This saves manufacturers crucial time and effort, setting new benchmarks for speed, reliability, and precision in medical device manufacturing.

ESSERT MicroFactory – customizable and future-proof medical device assembly
The ESSERT MicroFactory redefines standards in High Mix / Low Volume production: It offers elevated levels of flexibility, scalability, and efficiency.
Composed of multiple process modules, its flexible architecture can be easily tailored and reconfigured according to changing manufacturing needs, thanks to its Plug & Produce design. Furthermore, the MicroFactory can be designed right away to produce different product types, with changeover times below 15 minutes.
The seamless integration of manual workstations with automated processes ensures a straightforward workflow. Service-friendly design, the possibility to pre-validate individual modules, and scalability empower manufacturers to effortlessly address diverse production needs.
Beyond the assembly floor, the Process Control Center provides centralized command, delivering real-time oversight and data visualization. Regulatory compliance can be ensured, along with user-friendliness and transparency, when managing multiple production processes on a single medical device assembly line – the ESSERT MicroFactory.